At the 17th ISMPP virtual meet, I attended a session on Digital Therapeutics titled The digital therapeutics revolution: Are medical communications professionals prepared? Led by Randall Kaye, MD, ex CMO, at Click Therapeutics, a leader in digital healthcare, and Samuel Falsetti, Ph.D., EVP, Global Biotechnology; Practice Lead, at Medistrava Consulting, the session talked about the changing landscape of HealthTech and the nuances of prescription digital therapeutics (PDTs). Here’s the summary of all I learned at the session.
Summary/Discussion points:
Learning objectives of the session:
- Know how to differentiate prescription digital therapeutics (PDTs) from pharmaceuticals
- Understand the complexities of pivotal trial programs and regulatory approval for PDTs
- Recognize the key challenges/opportunities for Medical Affairs professionals in developing and executing medical communications for PDTs
Based on his experience at Click Therapeutics, Randall mentioned that it is important to educate people about DTs. “A Digital Therapeutic is Not an App”; while the patient/consumer may think of it as just an app, a lot of science and research is needed for the development of a true DT.
Randall explained the definition of a DT as follows: “It is more like a medical device to which you can make some changes through the course of its lifecycle”.

Randall explained the path from the development to the commercialization of a PDT with an example from Click Therapeutics. It goes through a similar cycle as that of a drug: development –> validation –> registration –> commercialization. He explained that clinically validating the application is a critical step; it is not just a few individuals testing the app for functionality. PDT, like a drug, goes through randomized control trials, and in place of a placebo, there is a mock intervention.
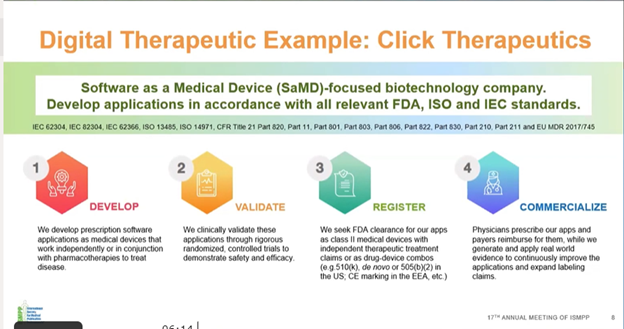
How does the development of a PDT differ from that of a medication or a device?
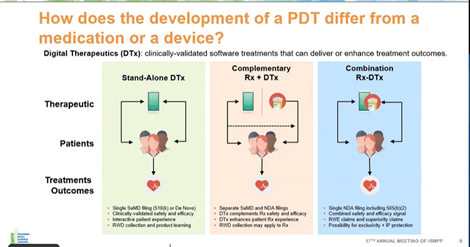
PDT can be used as a stand-alone therapy or in combination with conventional treatments such as pharmacological or in-person treatments; however, establishing the right paradigm for combination is very difficult, and it needs to be established that the combination would be better than the individual treatments.
When is the best time to initiate Medical Affairs activities for PDTs?
The answer is “as early as possible/NOW!” Medical Affairs teams need to focus on the audience and how they will understand the new modality and value of PDTs; understand how healthcare professionals (HCPs) will incorporate PDTs into their clinical practice; understand the primary and secondary efficacy data from pivotal trials and determine what other evidence needs to be presented; think about the target product profile and post-launch data collection methods to establish and validate the efficacy of this therapy; and so on. Research data should also be published as early as possible.
Why now?
DTs are very new and complex, and their use will involve changing standard therapeutic practices. Several aspects of audience behavior and knowledge gaps that exist also need to be considered, especially when a cognitive behavior-changing DT is prescribed. Patients may wonder why they are being prescribed a game instead of a drug, for example.
Just like for a prescription drug, the steps for developing a DT are quite time-consuming, including assessing and understanding the key communication pillars in a Medical Affairs plan, key target audiences, etc. To develop a best-in-class drug, Medical Affairs teams may follow a “normal timeline for a communication plan; however, for a DT, it is better to prepare a communication timeline as early as possible as it is new to the prescriber and is probably being used/prescribed for the first time.
Key challenges specific to PDTs:
- Launch and lifecycle management: The communication pillars and timeline planning for DT launch are mostly similar to those adopted for drug development. Lifecycle management is however very different for a DT. Drugs cannot be frequently altered based on feedback from patients. However, such changes can be made to a DT as it is a program that can be modified and adjusted based on information accrued. Depending on how the program is set up or the device is structured and where the data exist (cloud-based server, native on device, etc.), data can be aggregated and the effectiveness of the product can be observed in real time, by introducing coding-based formulation changes.
- Publications: There is a lot of misinformation about various apps, and this provides a huge opportunity to educate people about DTs. Early publications are necessary to help people understand how a DT is applied in a specific therapeutic area. It is advisable to assume that your HCP, payer, and patient audiences do not know what a DT is. As DTs are relatively new, published literature for DTs is scarcer than that for drugs. There are specific digital health and medicine journals that accept DT-related papers, and such publications are important when communicating with digital opinion leaders and people invested in digital health and therapeutics who want to learn about app-specific data and digital modalities. Some examples of such journals are Digital Health published by Nature and Digital Medicine published by the International Society for Digital Medicine. For each individual therapy, Medical Affairs teams should think about how to introduce a lexicon about PDTs. Moreover, clinical trials and treatment modalities should be introduced well ahead of launch since the readers may not be very familiar with PDTs in general; thus, when launch data are released, there is already a basic understanding of PDTs.
- Medical Information: The user base and the prescriber audience for PDTs are very varied and broad. Medical Information platforms for DTs have to cater to a much broader audience than specialists/sub-specialists and should include those involved in primary care, physicians, physician assistants, nurse practitioners, and clinical psychologists (in the case of DTs for neurological disorders).
- Field Medical: Scientific exchange should include several stakeholders who may be affiliated with a practice but may not necessarily be prescribers, for example, cognitive behavioral therapists; it is important for these stakeholders to thoroughly understand the product as well. Reactive scientific exchange with payers is also critical, and it is important to focus on lexicon and value narrative, considering that DTs are novel to the payer audience as well. In addition to possessing the necessary scientific qualifications, field medical staff for DTs should also be technologically savvy.
- Key opinion leader development: The prescribing/influencing stakeholders must have an appreciation and understanding of the role that technology plays in supporting and supplementing, rather than replacing, their practice.
- Real-world evidence/Health economics and outcomes research: By their nature, DTs generate huge amounts of data. Unlike in a clinical trial for a drug with fixed primary and secondary endpoints, the size and scale of real-world data generated by DTs help us explore multiple (and often contemporaneous) exploratory endpoints. Understanding these data is critical to determining the planning of evidence generation.

This article is part of a comprehensive report by Cactus Life Sciences on the Virtual 17th Annual Meeting of ISMPP. Click here to get the full report.
Share this post
About the author

Shyam Dattani
Shyam Dattani is Vice President, Business Development, at Cactus Life Sciences.













