Patient engagement is a big buzzword phrase these days. And while the medical publications and communications community has made progress in creating clear, non-technical content for patients, finding that content isn’t always easy. So why is finding plain language summaries (PLS) so difficult for patients?
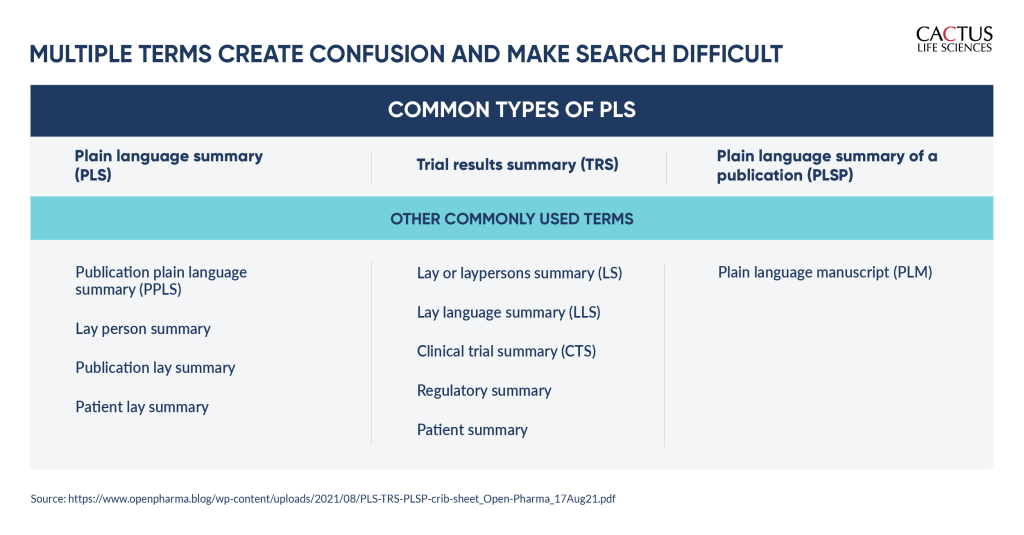
A recent review of biomedical research journals showed that almost all of them that published PLS made that content available for free. However, there were inconsistencies in terminology, location, and other factors, which could present challenges for lay audiences when searching for this content. For instance, PLS or trial results can be referred to by many different names, creating confusion instead of clarity among lay people. Many patients may not even know what a “plain language summary” is. Adding to the complexity, plain language content can be located in many different places, including alongside a scientific article, within the article itself, or as stand-alone content. Lastly, PLS commonly use non-intuitive terms that lay people have difficulty understanding. For example, terms used in biotech publications include “significance statement” and “author summary” to represent PLS, which may not be meaningful to lay readers and lead to them overlook that content.
Why are PLS important?
Medical research is currently in the public eye like never before. Patient engagement in the form of clear and understandable content is imperative to creating trust and partnership with HCPs, as more patients and caregivers seek out more control over their medical care. Clinical trial programs are a prime example of why this content is so vital. Here, patient ability to search, engage with, be aware of, and participate in clinical trial optimization is key. Despite concerns around direct-to-patient marketing and promotional regulations, building compliant PLS based on consensus guidelines around language and best practices is critical to the industry moving forward. Both the EU and the US regulatory agencies are making PLS a mandatory focal point for clinical trials, with the goal of improving understanding and participation.
PLS language standardization
PLS aren’t just for patients. In fact, expanding their use to include any stakeholder who could benefit from layperson language may increase usage and viability for many companies. Many organizations are working toward standardizing the way PLS are identified and formatted to improve accessibility. Recommendations for doing so include
- Using an icon to signpost PLS content
- Co-creating content with patient organizations to ensure clarity and use of proper terms
- Utilizing peer review of this content at the same time as the scientific content
- Plain text indexing in PubMed and other directories
- Linking to the source article
- Tagging with appropriate metadata and search keywords to improve discoverability
While plain language, text-only formats are recommended as the primary means of communicating with patients, it has been suggested that infographics and videos can further enhance understanding and supplement the main takeaways.
Creating compelling PLS
Medical Information is the obvious driver of patient-facing scientific content. However, most departments find themselves overwhelmed and ill-prepared to take on the task of creating PLS. That’s why finding the right scientific communications partner is so important. At Cactus Life Sciences, we have years of experience working with Medical Information teams at some of the largest pharmaceutical companies in the United States. From simplifying science to meet health literacy standards, to creating infographics to tell your story, to co-authorship collaborations between clinicians and patients, Cactus Life Sciences has a global array of multidisciplinary experts at your command. To find out more about how we can help you meet the demands of today’s patient-focused business challenges, contact us today.
Share this post
About the author

Laura Perry
Laura Perry is Vice President, Marketing, at Cactus Life Sciences.