Patient-centricity isn’t about the relationship. It’s about the relationshift.
The relationship between patients and the healthcare industry is undergoing a seismic shift. Industry-wide adoption of the patient-centric approach means patients are now involved at every possible stage in the drug research and development process. The traditional clinician-patient relationship has been redefined from HCP-dominated to patient-equal, and this is being reflected in peer review of research from study development through article publication.
Patient peer reviewers offer the industry a unique opportunity to see exactly how patients think pharmaceutical companies are doing in terms of addressing their actual needs through research. The goal is not to have patients comment on the scientific accuracy of the data, or its originality or importance to clinicians, but to communicate whether the research in question addresses an issue that is important to them as patients.
Patient peer reviewers: Who are they and what do they want?

Patient participation in clinical studies used to end far before the results were sent in to publications. That is all about to change. Patients are now more educated than ever and looking to contribute their experiences to the overall clinical study experience. Patient peer reviewers are particularly interested in co-designing trials and studies, co-authoring academic research papers, and joining trial management teams. They have lived with the disease themselves and have become experts in their condition. They want to leverage these real-world perceptions and experiences in ways that add value to today’s medical research and to the patient community it impacts. They see themselves as partners in the process, not just a means to an endpoint. Many patient reviewers start as patient advocates. These advocates undergo training to become peer reviewers, learning about the science behind the data through patient-/patient advocate-focused programs that disseminate the information in clear and understandable language and formats. They also learn how to interface with authors/researchers, clinicians, and publications, as well as the legalities of participation.
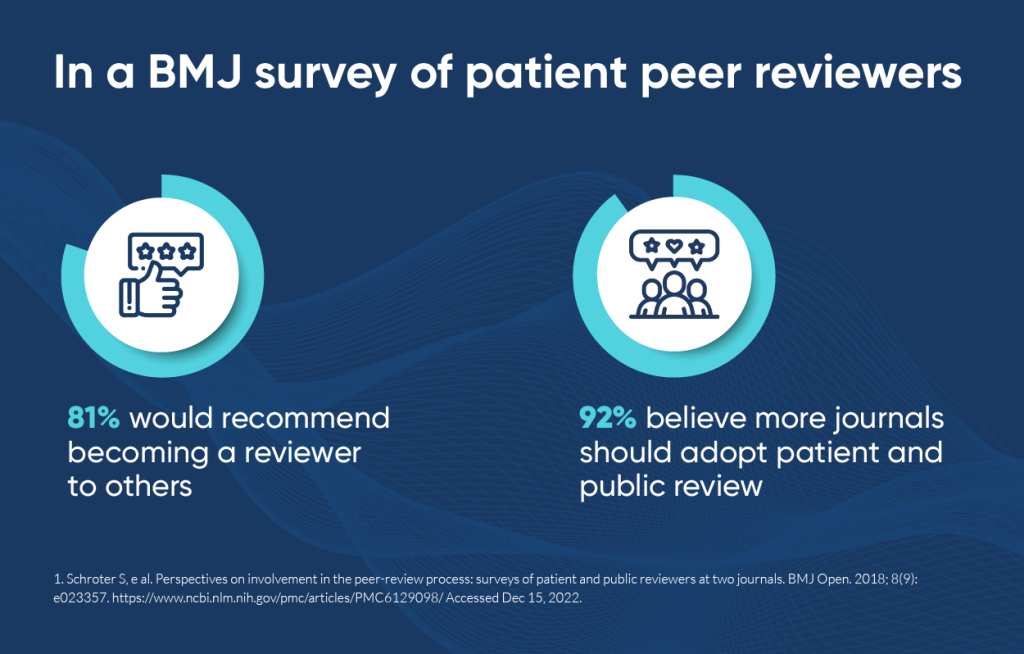
Identifying, training, and compensating patient peer reviewers
Identifying, training, and compensating patient peer reviewers can be done on an organizational level, or through associations that specialize in connecting companies with patient reviewers. For example, BMJ and Cochrane, actively recruit patient reviewers on their sites. To compensate patients for their time, each offers a complimentary 1-year subscription or membership to the reviewer. Alternatively, the American Association for Cancer Research provides resources to inform and connect potential patient reviewers with networks that may help them get started. Lastly, you can tap into the expertise of companies such as Cactus Life Sciences, that have worked extensively with pharmaceutical companies on a global scale to identify and partner with patient peer reviewers. To learn more about how Cactus Life Sciences can help you enhance your patient-centric initiatives, contact us.
Reference:
- Schroter S, e al. Perspectives on involvement in the peer-review process: surveys of patient and public reviewers at two journals. BMJ Open. 2018; 8(9): e023357. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6129098/ Accessed Dec 15, 2022.
Share this post
About the author

Laura Perry
Laura Perry is Vice President, Marketing, at Cactus Life Sciences.













